Effects and mechanisms of addition of different types of exogenous organic materials on priming effect of organic carbon in arable black soils
-
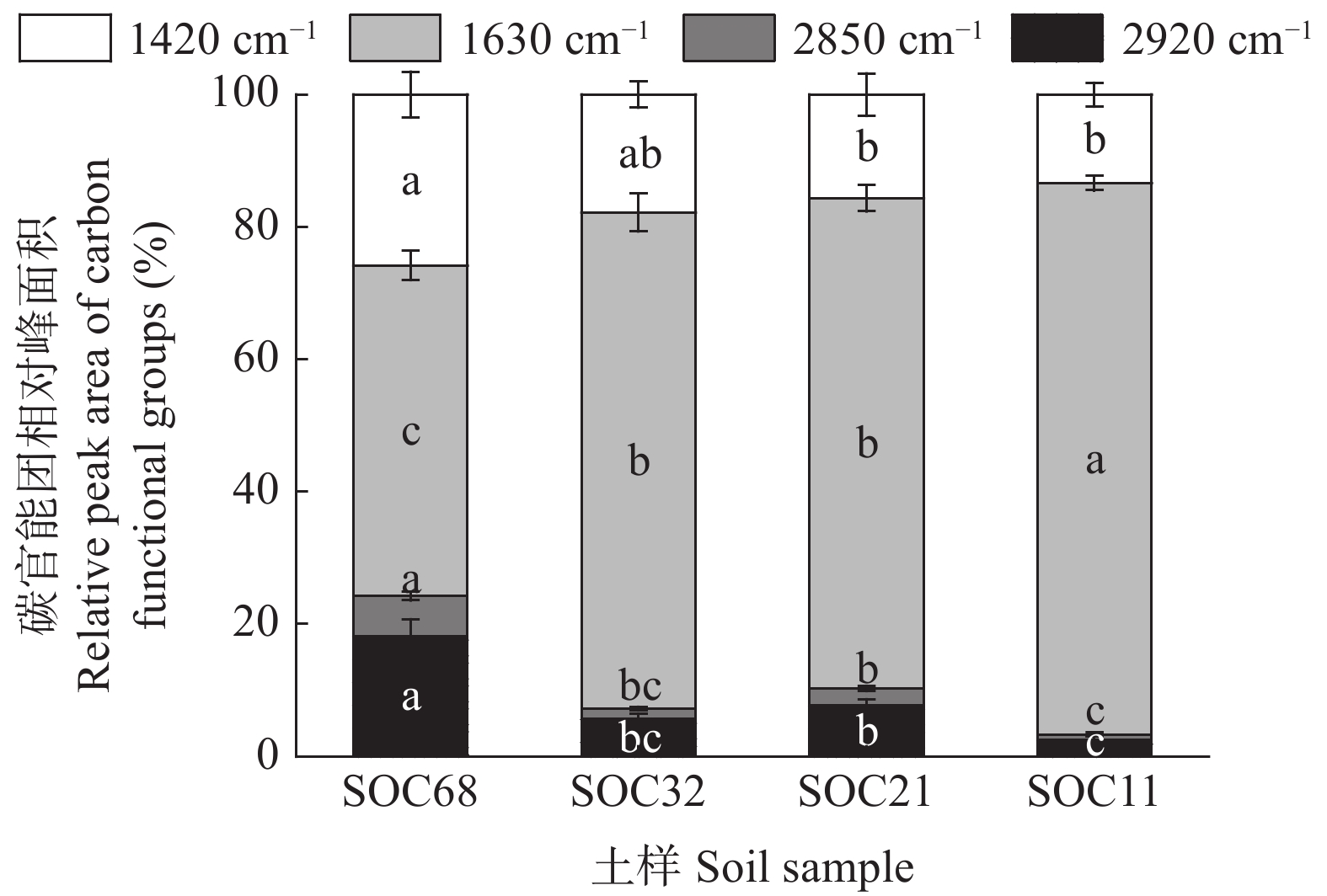
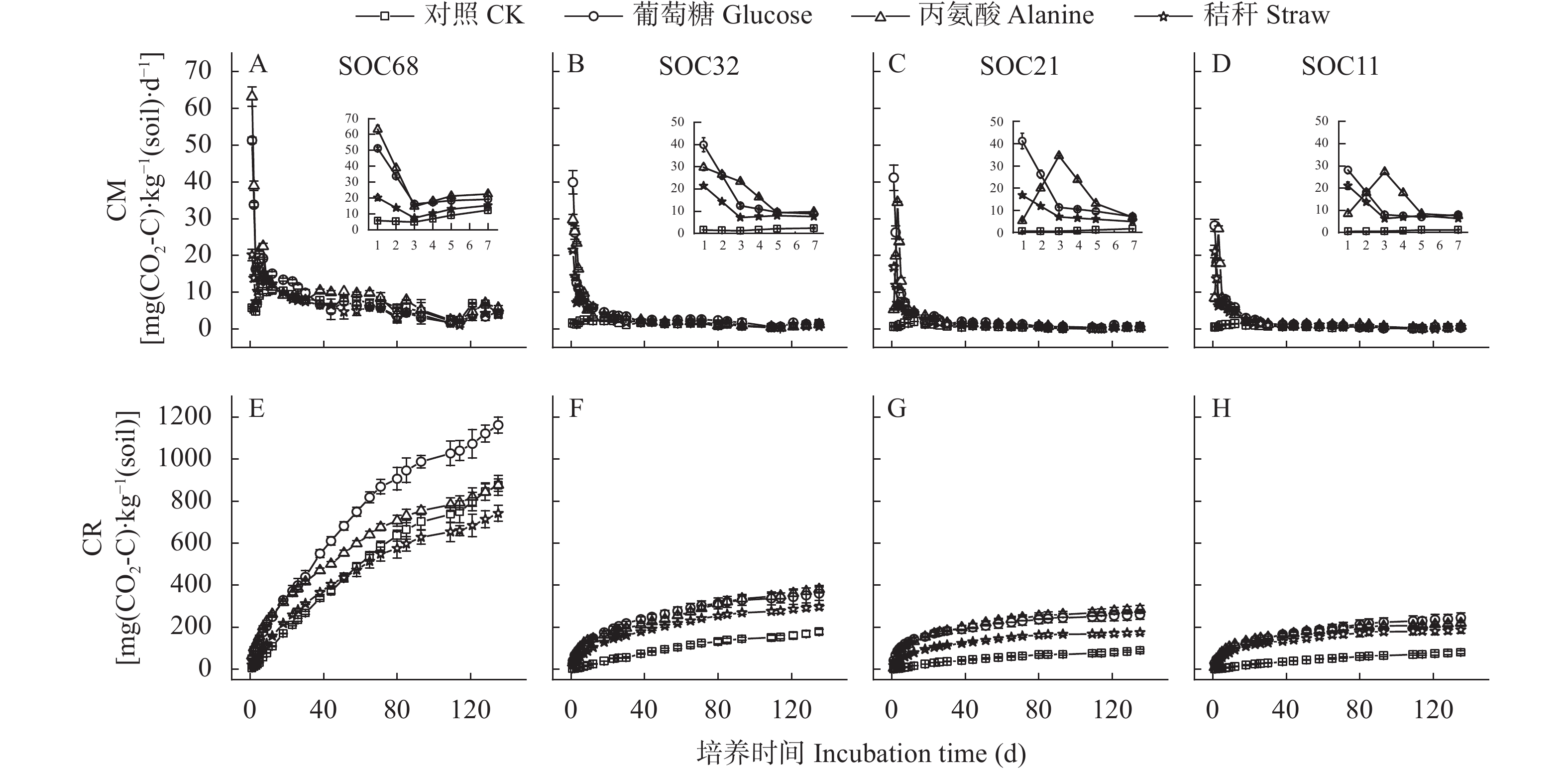
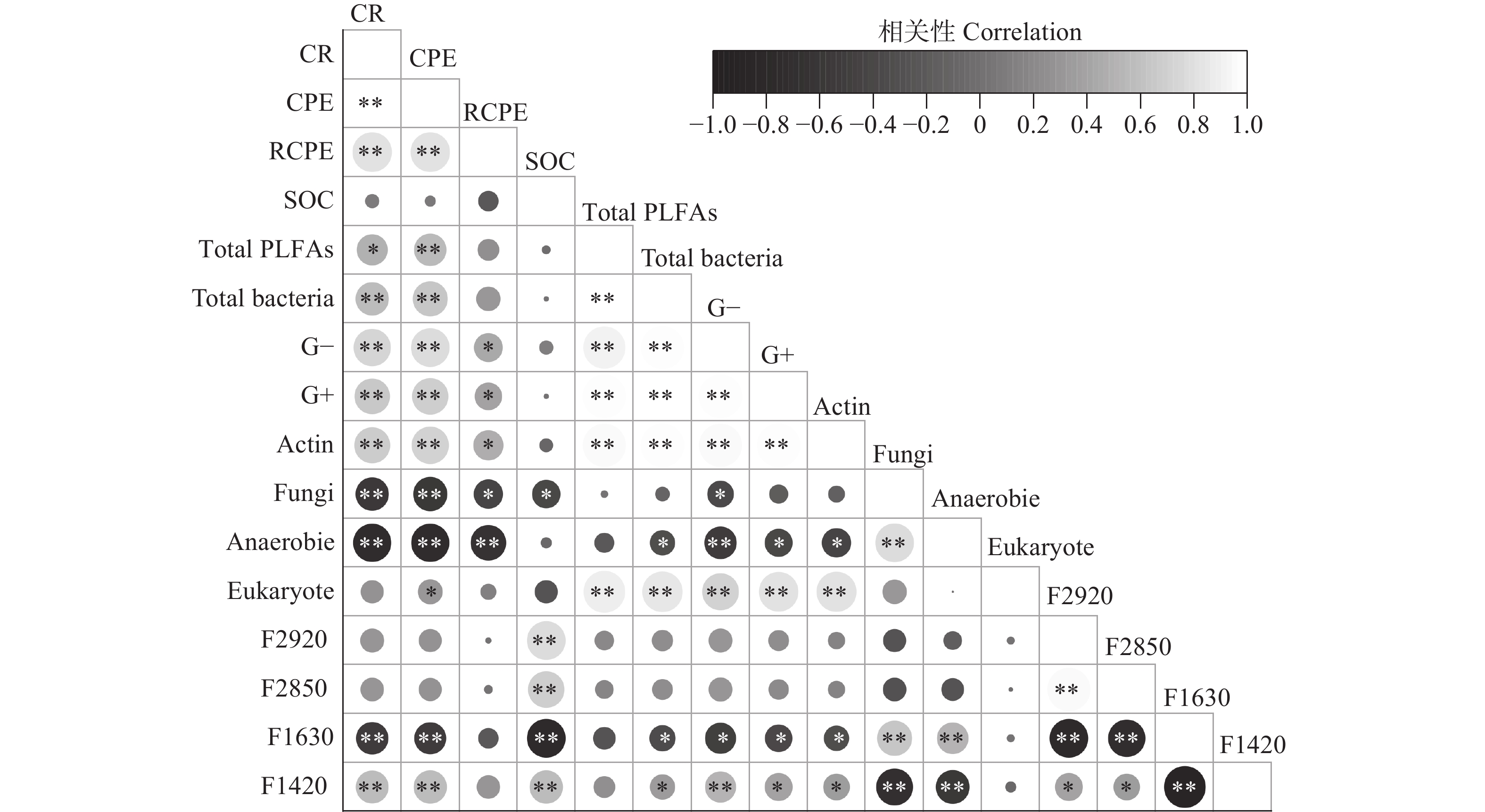
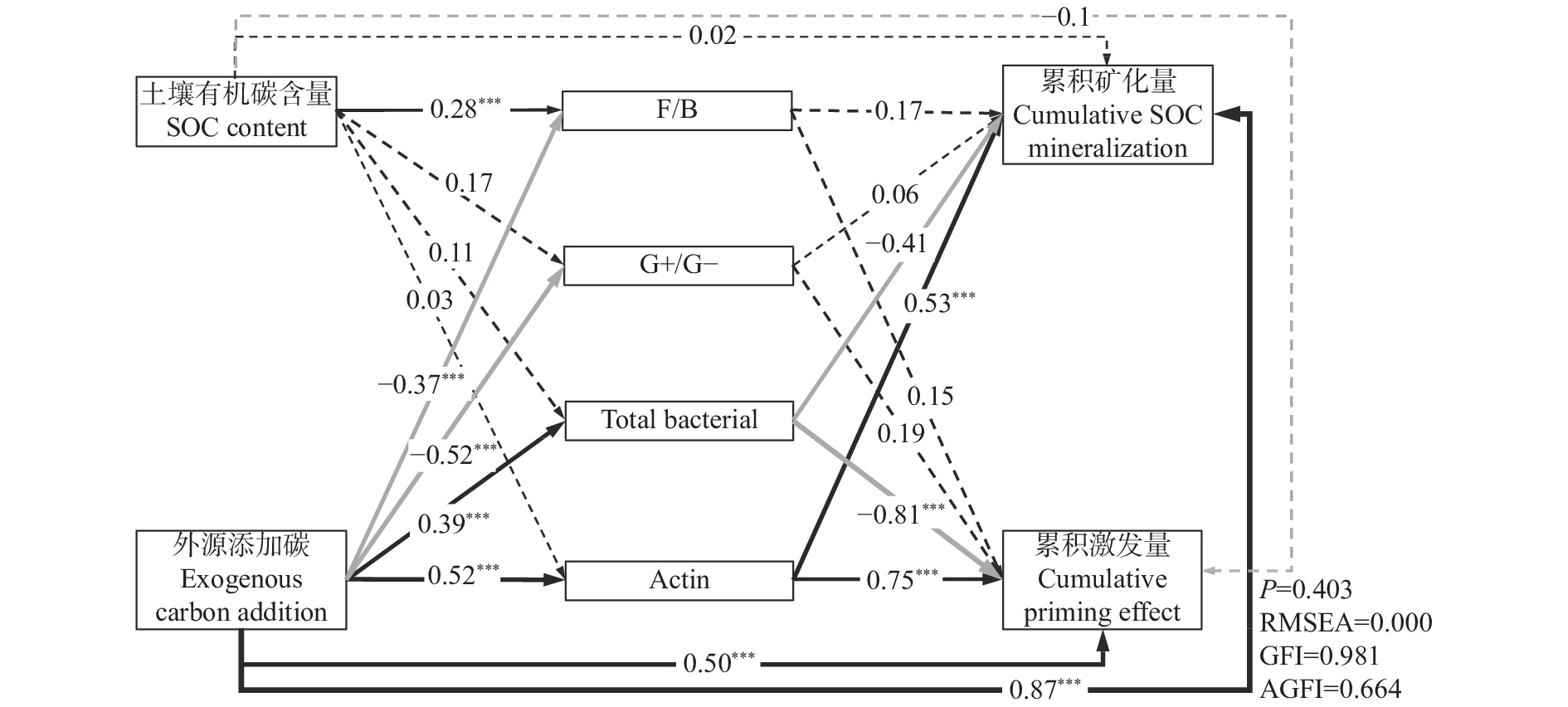
摘要: 外源有机物添加通过激发效应影响土壤有机碳(SOC)的矿化和稳定。目前研究较少考虑不同种类外源有机物添加对同一土壤类型不同SOC含量的土壤激发效应及作用机理。本研究以中国东北典型黑土带上4个不同SOC含量的表层农田黑土为研究对象, 向土壤中添加13C标记的玉米秸秆、葡萄糖和丙氨酸, 研究不同种类外源有机物添加对黑土SOC激发效应的影响及作用机理。研究发现, 与不添加外源有机物处理相比, 外源有机物添加均促进了4个不同SOC含量的农田土壤中SOC的矿化, 并引起土壤正激发效应。添加葡萄糖和丙氨酸处理的4个土壤在培养前30 d累积激发量均显著大于添加秸秆处理; 土壤本底SOC含量越高的土壤, 添加丙氨酸和葡萄糖产生的累积激发量越大, 但相对累积激发量越小。随着培养时间增加, 4个土壤的SOC矿化和激发效应降低并趋于稳定且处理间无显著差异。傅里叶红外光谱发现, 外源有机物添加后4个土壤脂肪族碳官能团的相对峰面积略有增加, 芳香族碳官能团峰面积略有下降, 土壤和外源有机物添加种类对脂肪族和芳香族碳官能团的影响均表现为土样>添加物种类。结构方程模型表明, 外源有机物添加产生的正激发效应主要受土壤细菌和放线菌磷脂脂肪酸的影响, 受土壤SOC含量影响较小。该研究结果表明, 外源有机物添加显著增加了农田黑土中k型微生物生长代谢, 促进了微生物对SOC中难分解组分的降解, 外源有机物与土壤自身有机碳分解的“共代谢”理论是本研究黑土产生正激发效应的主要作用机理。Abstract: The addition of exogenous organic matter (EOM) affects the mineralization and stabilization of soil organic carbon (SOC) via priming effects (PE). However, few studies have considered the effects of different EOM additions on PE in the same soil type with a gradient in SOC content. The underlying mechanisms have rarely been revealed, and related studies can provide in-depth insights into the microbial mechanisms that regulate carbon accumulation and stability in agricultural soils. It is crucial to predict dynamic changes in SOC and carbon pool stability in response to EOM inputs from different sources. Therefore, this study focused on topsoil with four SOC contents, which ranged from 10 g·kg−1 to nearly 70 g·kg−1, in the typical black-soil region of Northeast China, and aimed to investigate the effects and microbial mechanisms involving different types of EOM addition on PEs by adding 13C-labeled maize straw, glucose, and alanine to the soil. Compared to a control treatment without EOM, the addition of EOM promoted the mineralization of SOC in the four soils with different SOC contents. Specifically, glucose, alanine, and straw addition increased the cumulative mineralization of SOC by 50.88%–419.65%, 69.54%–409.48%, and 13.14%–321.43%, respectively. The addition of the three types of EOM also induced a positive PE in soils with different SOC contents. During the initial 30 days of incubation, the cumulative PEs in soils with different SOC contents under glucose and alanine addition treatments were considerably higher than those under straw addition treatment. Soils with higher SOC content exhibited greater cumulative mineralization and PEs with the addition of glucose and alanine, whereas their relative cumulative PEs were lower. SOC mineralization and PEs decreased and reached a stable state with incubation time in soils with different SOC contents. Fourier-Transform Infrared spectroscopy revealed a slight increase in the relative peak area of aliphatic carbon functional group and a slight decrease in the peak area of aromatic carbon group in soils with different contents of SOC after addition of EOM. The effects of SOC content on aliphatic and aromatic carbon functional groups were greater than those of the EOM type. Correlation analysis revealed that cumulative SOC mineralization and PEs were significantly positively correlated with total phospholipid fatty acids, biomass of total bacteria, gram-positive bacteria, gram-negative bacteria, and actinomycetes, with a peak area of aliphatic carbon at 1420 cm−1 (P<0.05). In addition, cumulative SOC mineralization and cumulative PEs were significantly negatively correlated with the biomass of fungi and anaerobic bacteria, with a peak area of aromatic carbon at 1630 cm−1 (P<0.05). Structural equation modeling indicated that the positive PE resulting from EOM addition was primarily influenced by bacterial and actinomycete phospholipid fatty acids in the soil, regardless of the SOC content of the four soil samples. These results demonstrated that EOM addition significantly increased the growth and metabolism of k-type microorganisms, such as gram-negative bacteria and actinomycetes, in arable black soil and promoted the decomposition of recalcitrant components in SOC. The “co-metabolism” theory, namely the co-decomposition of EOM and SOC, is considered as the primary mechanism behind the positive PE in black soil.
-
图 3 不同种类外源有机物添加对农田黑土有机碳的激发效应(A-D)、培养30 d的累积激发效应(E-H)和相对累积激发效应(I-L)的影响
Figure 3. Effects of addition of different types of exogenous organic materials on priming effect (A-D), cumulative priming effect (E-H) and relative cumulative priming effect (I-L) during the first 30 days of incubation of arable black soils with different soil organic carbon contents
表 1 试验前供试土壤基本理化性质和微生物生物量碳含量
Table 1 Physical-chemical properties and microbial biomass carbon content before incubation of the tested soil
土样
Soil sample经纬度
Longitude,
latitude总有机碳
Soil
organic carbon
(g·kg−1)全氮
Total
nitrogen (g·kg−1)pH 全磷
Total phosphorus (g·kg−1)全钾
Total
potassium (g·kg−1)有机磷
Olsen phosphorus
(mg·kg−1)速效钾
Available potassium (mg·kg−1)微生物生物
量碳
Microbial biomass carbon
(mg·kg−1)SOC68 48°15′N, 126°21′E 68.16±0.33a 5.65±0.02a 6.89±0.01a 1.73±0.00a 16.2±0.9b 104.4±2.32a 628.8±15.9a 549.72±75.45a SOC32 47°27′N, 126°55′E 31.50±0.22b 2.56±0.01b 5.63±0.05b 1.06±0.02b 18.4±0.4a 44.5±3.15c 138.8±0.8c 353.10±4.13b SOC21 44°59′N, 126°21′E 20.59±0.11c 2.21±0.12c 5.14±0.01c 0.97±0.00c 18.4±0.3a 52.7±2.09b 159.0±8.4b 117.99±1.13d SOC11 43°12′N, 124°11′E 11.12±0.07d 1.07±0.01d 5.17±0.01c 0.46±0.00d 17.5±2.8ab 15.6±0.99d 106.2±19.3d 182.99±23.58c SOC68、SOC32、SOC21和SOC11分别表示北安、海伦、榆树和四平采集的土样。同列不同小写字母表示4个土样在P<0.05水平差异显著。SOC68, SOC32, SOC21 and SOC11 represent soils sampled from Bei’an, Hailun, Yushu and Siping, respectively. Different lowercase letters in the same column indicate significant differences among different soil samples (P<0.05). 表 2 土壤有机碳含量和外源有机物种类对土壤有机碳累积矿化量、累积激发量和有机碳官能团相对峰面积影响的双因素方差分析
Table 2 Two-way ANOVA for the influences of soil organic carbon (SOC) content, addition of different types of exogenous organic materials and their interactions on cumulative SOC mineralization, cumulative priming effect and infrared relative peak area of SOC functional groups
要素
ElementsS M S×M P Eta P Eta P Eta 培养135 d累积矿化量
Cumulative SOC mineralization after 135 d incubation<0.001 0.474 <0.001 0.819 <0.001 0.679 培养30 d累积激发量
Cumulative priming effect after 30 d incubation<0.001 0.568 <0.001 0.215 0.021 0.147 培养30 d相对累积激发量
Relative cumulative priming effect after incubation for 30 d<0.001 0.624 <0.001 0.347 0.028 0.165 F2920 <0.001 0.801 0.033 0.09 0.017 0.189 F2850 <0.001 0.777 0.067 0.072 0.001 0.255 F1630 <0.001 0.924 0.883 0.003 0.233 0.103 F1420 <0.001 0.831 0.039 0.086 0.001 0.264 S和M分别表示土壤有机碳含量和添加外源有机物种类。P和Eta分别表示P值和偏Eta方。F2920、F2850、F1630和F1420分别表示有机碳官能团在2920 cm−1、2850 cm−1、1630 cm−1和1420 cm−1处的相对吸收峰面积。S and M represent SOC content and type of exogenous organic material addition, respectively. P and Eta represent P value and partial Eta squared, respectively. F2920, F2850, F1630 and F1420 indicate the relative peak absorption areas of organic carbon functional groups at 2920, 2850, 1630 and 1420 cm−1, respectively. -
[1] GOMEZ-CASANOVAS N, MATAMALA R, COOK D R, et al. Net ecosystem exchange modifies the relationship between the autotrophic and heterotrophic components of soil respiration with abiotic factors in prairie grasslands[J]. Global Change Biology, 2012, 18(8): 2532−2545 doi: 10.1111/j.1365-2486.2012.02721.x
[2] KUZYAKOV Y, FRIEDEL J K, STAHR K. Review of mechanisms and quantification of priming effects[J]. Soil Biology and Biochemistry, 2000, 32(11/12): 1485−1498
[3] KEUPER F, WILD B, KUMMU M, et al. Carbon loss from northern circumpolar permafrost soils amplified by rhizosphere priming[J]. Nature Geoscience, 2020, 13(8): 560−565 doi: 10.1038/s41561-020-0607-0
[4] SAYER E J, HEARD M S, GRANT H K, et al. Soil carbon release enhanced by increased tropical forest litterfall[J]. Nature Climate Change, 2011, 1(6): 304−307 doi: 10.1038/nclimate1190
[5] BASTIDA F, GARCÍA C, FIERER N, et al. Global ecological predictors of the soil priming effect[J]. Nature Communications, 2019, 10(1): 3481 doi: 10.1038/s41467-019-11472-7
[6] CHEN L Y, LIU L, QIN S Q, et al. Regulation of priming effect by soil organic matter stability over a broad geographic scale[J]. Nature Communications, 2019, 10(1): 1−10 doi: 10.1038/s41467-018-07882-8
[7] YAN S B, YIN L M, DIJKSTRA F A, et al. Priming effect on soil carbon decomposition by root exudate surrogates: a meta-analysis[J]. Soil Biology and Biochemistry, 2023, 178: 108955 doi: 10.1016/j.soilbio.2023.108955
[8] PERVEEN N, BAROT S, MAIRE V, et al. Universality of priming effect: an analysis using thirty-five soils with contrasted properties sampled from five continents[J]. Soil Biology and Biochemistry, 2019, 134: 162−171 doi: 10.1016/j.soilbio.2019.03.027
[9] SUN Z L, LIU S G, ZHANG T A, et al. Priming of soil organic carbon decomposition induced by exogenous organic carbon input: a meta-analysis[J]. Plant and Soil, 2019, 443(1): 463−471
[10] GUENET B, CAMINO-SERRANO M, CIAIS P, et al. Impact of priming on global soil carbon stocks[J]. Global Change Biology, 2018, 24(5): 1873−1883 doi: 10.1111/gcb.14069
[11] 甘子莹, 王浩, 丁驰, 等. 亚热带森林不同植物及器官来源的可溶性有机质输入对土壤激发效应的影响及其作用机理[J]. 植物生态学报, 2022, 46(7): 797−810 doi: 10.17521/cjpe.2021.0288 GAN Z Y, WANG H, DING C, et al. Effects of dissolved organic matter derived from different plant and tissues in a subtropical forest on soil priming effect and the underlying mechanisms[J]. Chinese Journal of Plant Ecology, 2022, 46(7): 797−810 doi: 10.17521/cjpe.2021.0288
[12] TIAN Q X, JIANG Q H, ZHAO R D, et al. Microbial properties control soil priming and exogenous carbon incorporation along an elevation gradient[J]. Geoderma, 2023, 431: 116343 doi: 10.1016/j.geoderma.2023.116343
[13] LIN Q, DE VRIEZE J, FANG X Y, et al. Labile carbon feedstocks trigger a priming effect in anaerobic digestion: an insight into microbial mechanisms[J]. Bioresource Technology, 2022, 344: 126243 doi: 10.1016/j.biortech.2021.126243
[14] ZHOU J, QIAO N, ZHU T B, et al. Native soil labile organic matter influences soil priming effects[J]. Applied Soil Ecology, 2023, 182: 104732 doi: 10.1016/j.apsoil.2022.104732
[15] DIJKSTRA F A, HUTCHINSON G L, REEDER J D, et al. Elevated CO2, but not defoliation, enhances N cycling and increases short-term soil N immobilization regardless of N addition in a semiarid grassland[J]. Soil Biology and Biochemistry, 2011, 43(11): 2247−2256 doi: 10.1016/j.soilbio.2011.07.017
[16] HAMER U, MARSCHNER B. Priming effects in soils after combined and repeated substrate additions[J]. Geoderma, 2005, 128(1/2): 38−51
[17] LUO Y Q, ZHAO X Y, ANDRÉN O, et al. Artificial root exudates and soil organic carbon mineralization in a degraded sandy grassland in Northern China[J]. Journal of Arid Land, 2014, 6(4): 423−431 doi: 10.1007/s40333-014-0063-z
[18] LIANG C, SCHIMEL J P, JASTROW J D. The importance of anabolism in microbial control over soil carbon storage[J]. Nature Microbiology, 2017, 2(8): 1−6
[19] 梁超, 朱雪峰. 土壤微生物碳泵储碳机制概论[J]. 中国科学(地球科学), 2021, 51(5): 680−695 doi: 10.1360/SSTe-2020-0213 LIANG C, ZHU X F. Introduction to carbon storage mechanism of soil microbial carbon pump[J]. Scientia Sinica (Terrae), 2021, 51(5): 680−695 doi: 10.1360/SSTe-2020-0213
[20] BLAGODATSKAYA Е, KUZYAKOV Y. Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review[J]. Biology and Fertility of Soils, 2008, 45(2): 115−131 doi: 10.1007/s00374-008-0334-y
[21] YU Z, CHEN L, PAN S, et al. Feedstock determines biochar-induced soil priming effects by stimulating the activity of specific microorganisms[J]. European Journal of Soil Science, 2018, 69(3): 521−534 doi: 10.1111/ejss.12542
[22] ZHOU S, WANG J Y, CHEN L, et al. Microbial community structure and functional genes drive soil priming effect following afforestation[J]. Science of the Total Environment, 2022, 825: 153925 doi: 10.1016/j.scitotenv.2022.153925
[23] LI C, XIAO C W, LI M X, et al. The quality and quantity of SOM determines the mineralization of recently added labile C and priming of native SOM in grazed grasslands[J]. Geoderma, 2023, 432: 116385 doi: 10.1016/j.geoderma.2023.116385
[24] VANCE E D, BROOKES P C, JENKINSON D S. An extraction method for measuring soil microbial biomass C[J]. Soil Biology and Biochemistry, 1987, 19(6): 703−707 doi: 10.1016/0038-0717(87)90052-6
[25] 盛明, 龙静泓, 雷琬莹, 等. 秸秆还田对黑土团聚体内有机碳红外光谱特征的影响[J]. 土壤与作物, 2020, 9(4): 355−366 doi: 10.11689/j.issn.2095-2961.2020.04.004 SHENG M, LONG J H, LEI W Y, et al. Effect of straw returning on the characteristics of Fourier Infrared Spectroscopy organic carbon within aggregates in a Mollisols[J]. Soil and Crop, 2020, 9(4): 355−366 doi: 10.11689/j.issn.2095-2961.2020.04.004
[26] TIVET F, DE MORAES SÁ J C, LAL R, et al. Assessing humification and organic C compounds by laser-induced fluorescence and FTIR spectroscopies under conventional and no-till management in Brazilian Oxisols[J]. Geoderma, 2013, 207/208: 71−81 doi: 10.1016/j.geoderma.2013.05.001
[27] BOSSIO D A, SCOW K M, GUNAPALA N, et al. Determinants of soil microbial communities: effects of agricultural management, season, and soil type on phospholipid fatty acid profiles[J]. Microbial Ecology, 1998, 36(1): 1−12 doi: 10.1007/s002489900087
[28] LI N, YAO S H, YOU M Y, et al. Contrasting development of soil microbial community structure under no-tilled perennial and tilled cropping during early pedogenesis of a Mollisol[J]. Soil Biology and Biochemistry, 2014, 77: 221−232 doi: 10.1016/j.soilbio.2014.07.002
[29] DAWSON T E, MAMBELLI S, PLAMBOECK A H, et al. Stable isotopes in plant ecology[J]. Annual Review of Ecology and Systematics, 2002, 33: 507−559 doi: 10.1146/annurev.ecolsys.33.020602.095451
[30] YOU M, HAN X, CHEN X, et al. Effect of reduction of aggregate size on the priming effect in a Mollisol under different soil managements[J]. European Journal of Soil Science, 2019, 70(4): 765−775
[31] BIMÜLLER C, KREYLING O, KÖLBL A, et al. Carbon and nitrogen mineralization in hierarchically structured aggregates of different size[J]. Soil and Tillage Research, 2016, 160: 23−33 doi: 10.1016/j.still.2015.12.011
[32] BERNAL B, MCKINLEY D C, HUNGATE B A, et al. Limits to soil carbon stability; deep, ancient soil carbon decomposition stimulated by new labile organic inputs[J]. Soil Biology and Biochemistry, 2016, 98: 85−94 doi: 10.1016/j.soilbio.2016.04.007
[33] TIAN J, PAUSCH J, YU G R, et al. Aggregate size and glucose level affect priming sources: a three-source-partitioning study[J]. Soil Biology and Biochemistry, 2016, 97: 199−210 doi: 10.1016/j.soilbio.2016.03.013
[34] 张叶叶, 莫非, 韩娟, 等. 秸秆还田下土壤有机质激发效应研究进展[J]. 土壤学报, 2021, 58(6): 1381−1392 doi: 10.11766/trxb202006260259 ZHANG Y Y, MO F, HAN J, et al. Research progress on the native soil carbon priming after straw addition[J]. Acta Pedologica Sinica, 2021, 58(6): 1381−1392 doi: 10.11766/trxb202006260259
[35] SIX J, FREY S D, THIET R K, et al. Bacterial and fungal contributions to carbon sequestration in agroecosystems[J]. Soil Science Society of America Journal, 2006, 70(2): 555−569 doi: 10.2136/sssaj2004.0347
[36] MASON-JONES K, SCHMÜCKER N, KUZYAKOV Y. Contrasting effects of organic and mineral nitrogen challenge the N-Mining Hypothesis for soil organic matter priming[J]. Soil Biology and Biochemistry, 2018, 124: 38−46 doi: 10.1016/j.soilbio.2018.05.024
[37] LIAN T X, WANG G H, YU Z H, et al. Bacterial communities incorporating plant-derived carbon in the soybean rhizosphere in Mollisols that differ in soil organic carbon content[J]. Applied Soil Ecology, 2017, 119: 375−383 doi: 10.1016/j.apsoil.2017.07.016
[38] 廖畅. 外源碳氮输入对不同纬度森林土壤有机碳矿化和固持的影响[D]. 武汉: 中国科学院大学(中国科学院武汉植物园), 2020 LIAO C. Effects of exogenous carbon and nitrogen input on mineralization and fixation of organic carbon in forest soils at different latitudes[D]. Wuhan: University of Chinese Academy of Sciences (Wuhan Botanical Garden, Chinese Academy of Sciences), 2020
[39] 刘本娟, 谢祖彬, 刘琦, 等. 生物质炭引起的土壤碳激发效应与土壤理化特性的相关性[J]. 土壤, 2021, 53(2): 343−353 LIU B J, XIE Z B, LIU Q, et al. Correlation between biochar-induced carbon priming effect in soils and soil physiochemical properties[J]. Soils, 2021, 53(2): 343−353
[40] SHAHBAZ M, KUZYAKOV Y, SANAULLAH M, et al. Microbial decomposition of soil organic matter is mediated by quality and quantity of crop residues: mechanisms and thresholds[J]. Biology and Fertility of Soils, 2017, 53(3): 287−301 doi: 10.1007/s00374-016-1174-9
[41] BALDOCK J A, OADES J M, VASSALLO A M, et al. Incorporation of uniformly labeled 13C glucose carbon into the organic fraction of a soil-carbon balance and CP MAS 13C NMR Measurements[J]. Soil Research, 1989, 27(4): 725 doi: 10.1071/SR9890725
[42] LUNDBERG P, EKBLAD A, NILSSON M. 13C NMR spectroscopy studies of forest soil microbial activity: glucose uptake and fatty acid biosynthesis[J]. Soil Biology and Biochemistry, 2001, 33(4/5): 621−632
[43] 张月玲. 高纬度土壤剖面有机碳的稳定性及其对土地利用变化的响应[D]. 北京: 中国农业科学院, 2019 ZHANG Y L. Stability of organic carbon in high latitude soil profile and its response to land use change[D]. Beijing: Chinese Academy of Agricultural Sciences, 2019
[44] ZHANG Y L, YAO S H, MAO J D, et al. Chemical composition of organic matter in a deep soil changed with a positive priming effect due to glucose addition as investigated by 13C NMR spectroscopy[J]. Soil Biology and Biochemistry, 2015, 85: 137−144 doi: 10.1016/j.soilbio.2015.03.013
[45] KEILUWEIT M, BOUGOURE J J, NICO P S, et al. Mineral protection of soil carbon counteracted by root exudates[J]. Nature Climate Change, 2015, 5(6): 588−595 doi: 10.1038/nclimate2580
[46] BALDOCK J A, OADES J M, VASSALLO A M, et al. Solid-state CP/MAS 13C NMR analysis of bacterial and fungal cultures isolated from a soil incubated fith glucose[J]. Soil Research, 1990, 28(2): 213 doi: 10.1071/SR9900213
[47] LEI W Y, PAN Q, TENG P J, et al. How does soil organic matter stabilize with soil and environmental variables along a black soil belt in Northeast China? An explanation using FTIR spectroscopy data[J]. CATENA, 2023, 228: 107152 doi: 10.1016/j.catena.2023.107152
[48] 石含之, 赵沛华, 黄永东, 等. 秸秆还田对土壤有机碳结构的影响[J]. 生态环境学报, 2020, 29(3): 536−542 SHI H Z, ZHAO P H, HUANG Y D, et al. Effect of straw mulching on soil organic carbon structure[J]. Ecology and Environment Sciences, 2020, 29(3): 536−542
[49] 彭义, 解宏图, 李军, 等. 免耕条件下不同秸秆覆盖量的土壤有机碳红外光谱特征[J]. 中国农业科学, 2013, 46(11): 2257−2264 PENG Y, XIE H T, LI J, et al. Effect of no-tillage with different stalk mulching on soil organic carbon and mid-infrared spectral characteristics[J]. Scientia Agricultura Sinica, 2013, 46(11): 2257−2264
[50] 朱雪峰, 张春雨, 郝艳杰, 等. 玉米秸秆覆盖还田量对免耕土壤有机碳中红外光谱特征的影响[J]. 应用生态学报, 2021, 32(8): 2685−2692 ZHU X F, ZHANG C Y, HAO Y J, et al. Effects of corn stover mulch quantity on mid-infrared spectroscopy of soil organic carbon in a no-tillage agricultural ecosystem[J]. Chinese Journal of Applied Ecology, 2021, 32(8): 2685−2692
[51] BLAGODATSKAYA E V, BLAGODATSKY S A, ANDERSON T H, et al. Priming effects in Chernozem induced by glucose and N in relation to microbial growth strategies[J]. Applied Soil Ecology, 2007, 37(1/2): 95−105





 下载:
下载: